

We have published our first Product Vigilance Report providing an introduction of our Product Vigilance programme*.
Product Vigilance (PV) encompasses the science and activities involved in detecting, assessing, understanding, and preventing adverse events associated with our Smokeless Product** portfolio.
This is not merely a regulatory function but a core tenet of our corporate integrity and our purpose to build A Better Tomorrow™ by reducing the health impact of our business. As a manufacturer of tobacco and nicotine products, we recognise the importance of monitoring and managing risks across the entire product lifecycle.
Our ambition in Product Vigilance is integral to BAT’s vision of Building a Smokeless World, by supporting the development and stewardship of innovative alternatives that meet product standards and are responsibly monitored. As these new product categories represent a critical shift away from traditional combustibles, our resources dedicated to post-launch monitoring have grown increasingly important.
Our Product Vigilance strategy, Vigilance360, follows three dimensions:
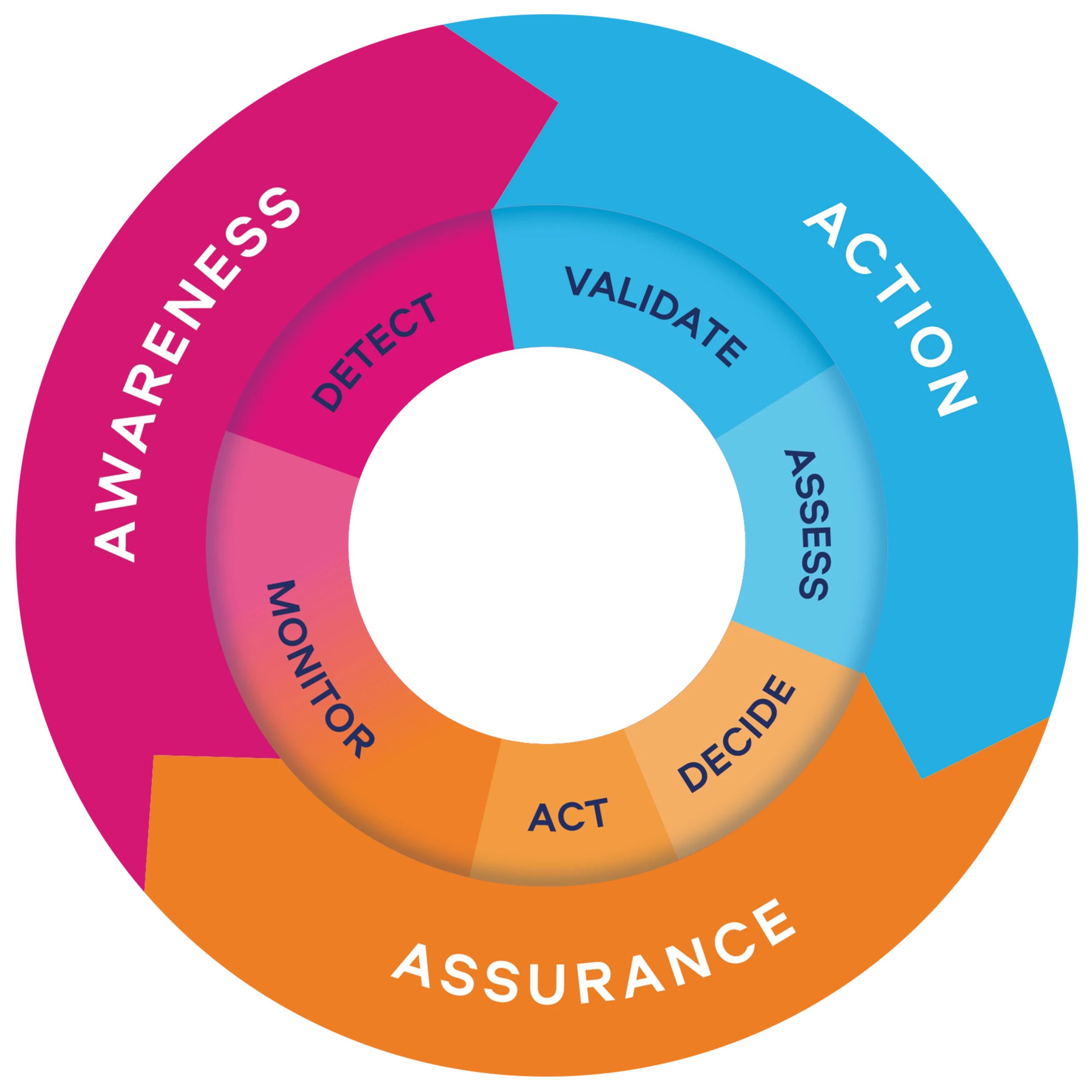
Our globally coordinated approach is supported by subject matter experts and vigilance ambassadors across careline centres worldwide. Consistency, regulatory alignment, and vigilance education enable compliance and scalability, creating an opportunity to make a real impact across the industry in the area of postmarket surveillance.
Disclaimer's
*For products sold in the U.S. by Reynolds American Inc.’s Operating Companies, procedures and data are managed under a dedicated product vigilance programme at subsidiary companies of Reynolds American Inc. For this reason, when this report discusses specific product vigilance procedures and related data, such procedures and data exclude the US.
**For the purpose of this publication, references to data on BAT's Smokeless Products do not include smoking cessation devices or other products licensed for Nicotine Replacement Therapy. With the exception of certain oral nicotine products, BAT's Smokeless Products are not smoking cessation devices and are not marketed for that purpose.