Global THR Science: Stakeholders and Key Research Questions
Tobacco Harm Reduction (THR) involves adult smokers who would otherwise continue smoking moving down the risk continuum towards less risky*† products than traditional cigarettes.
Figure 1. Key THR science stakeholders and their position related to tobacco control
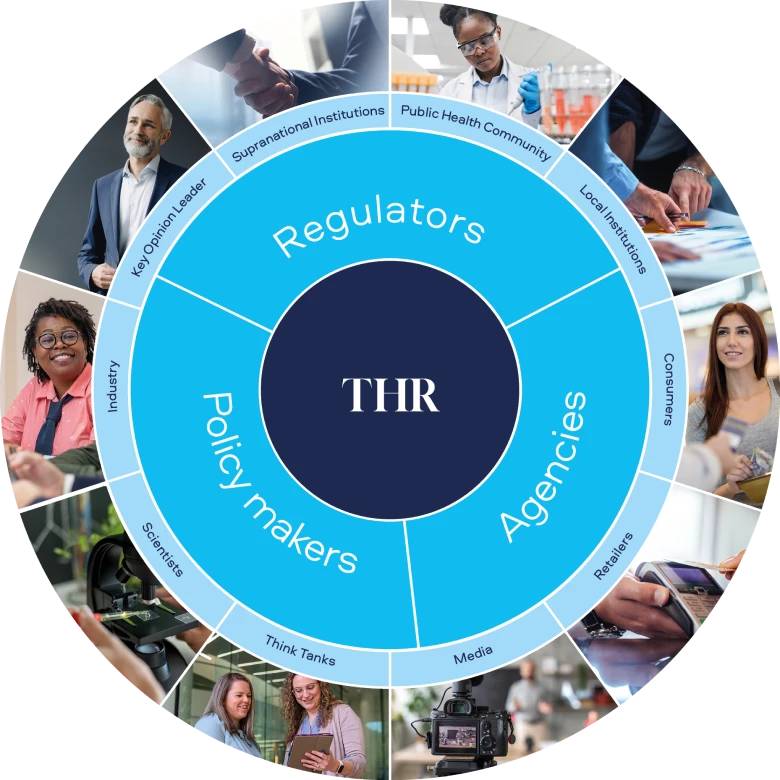
A growing body of global science supports the role of Smokeless Products, yet despite the tradition of harm reduction in public health, the regulatory and political landscape for THR remains complex (Figure 1). A big opportunity exists to bring scientific stakeholders together to unite and align in moving the public health strategy on tobacco forward.
Questions for THR
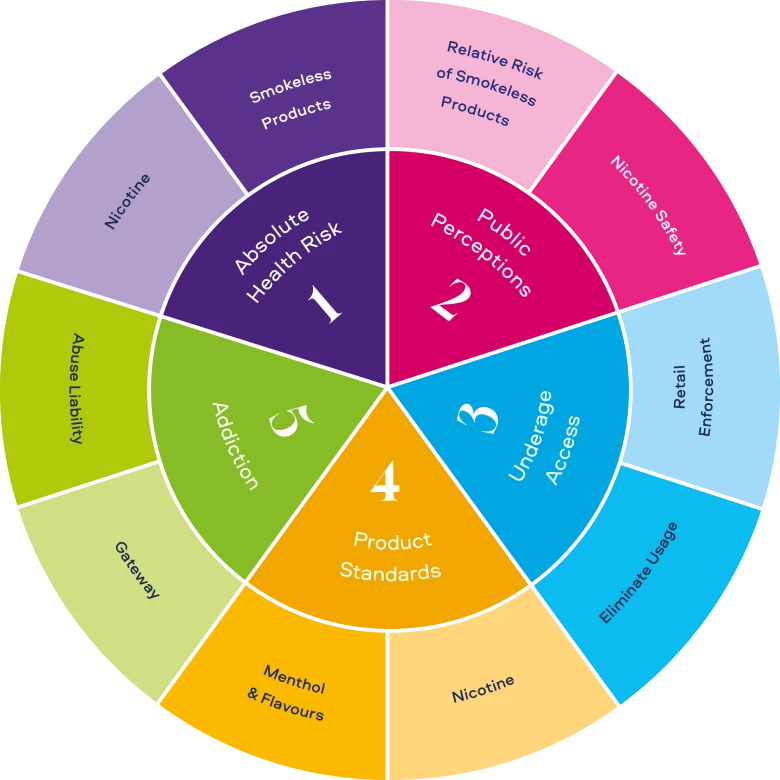
Tobacco and nicotine product science has evolved rapidly in the past decade, driven by the diversification of the tobacco industry. In turn, this has driven an equally rapid evolution of tobacco and nicotine product science, conducted by those outside the industry. Through conducting this science, both industry and non-industry scientists are looking for answers to key questions related to THR. These questions can be grouped into five main categories (Figure 2) and include:
ABSOLUTE HEALTH RISK
- What is the risk of tobacco and nicotine Smokeless Products on all users (smokers and non-smokers)?
- What are the health risks of nicotine use?
PUBLIC PERCEPTIONS
- Does the public understand the relative risk of tobacco and nicotine Smokeless Products compared to cigarettes?
- Does the public understand the risks of nicotine?
UNDERAGE ACCESS
- How can retail practices better prevent underage access to tobacco and nicotine Smokeless Products?
- What are the best methods to eliminate underage usage of tobacco and nicotine Smokeless Products?
PRODUCT STANDARDS
- What standards should be applied to nicotine in tobacco and nicotine Smokeless Products?
- What role do menthol and flavours play in encouraging adult smokers who would otherwise continue to smoke to switch completely to tobacco and nicotine Smokeless Products?
ADDICTION
- Do tobacco and nicotine Smokeless Products serve as a gateway to cigarette smoking?
- What are the abuse liability risks of tobacco and nicotine Smokeless Products relative to cigarettes?
Key Research Questions
Figure 2. Summation of key questions from our analysis of U.S/EU/other regulatory bodies
Footnotes
* Based on the weight of evidence and assuming a complete switch from cigarette smoking. These products are not risk free and are addictive.
† Our Vapour product Vuse (including Alto, Solo, Ciro and Vibe), and certain products, including Velo, Grizzly, Kodiak, and Camel Snus, which are sold in the U.S., are subject to FDA regulation and no reduced-risk claims will be made as to these products without agency clearance.
Sign up for more exclusive the Omni™ content
-
 Smokeless Products: An Introduction
Smokeless Products: An Introduction -
 01. Our Vision: A Need for Reappraisal
01. Our Vision: A Need for Reappraisal -
 02. The Big Questions
02. The Big Questions -
 03. Impact of Smoking
03. Impact of Smoking -
 04. Our Smokeless Science
04. Our Smokeless Science -
 05. Our Smokeless Products
05. Our Smokeless Products -
 06. THR: A Global Transformation
06. THR: A Global Transformation -
 07. THR: Global Regulation
07. THR: Global Regulation -
 08. THR: Scientific Engagement
08. THR: Scientific Engagement -
 09. Our Future Outlook
09. Our Future Outlook -
 10. References
10. References -
 11. Our Published THR Science
11. Our Published THR Science
