

Our transformation is underpinned by our purpose of reducing the health impact of our business.
To enable our transformation, we strive to encourage adult smokers, who would otherwise continue to smoke, to switch to reduced-risk*† Smokeless Products. Our focus continues to be on finding and developing new innovations and products and establishing data-based insights that evolve our innovation strategy to create products that could reduce the burden on global public health.
We are dedicated to innovation through every thread of the company, and we believe that success and longevity will be achieved through a science-based transformation.
Over the last decade, we have embedded the innovation of Smokeless Products in our organisation. This ensures the correct behaviours, structures and practices are in place to enable innovation to thrive. Added to that has been the evolution of our technical capabilities to enable the development of consumer loved products and the increased capabilities to scientifically assess their impact on public health.

We are in our eighth decade of research and development having established R&D sites in the U.S. and UK in the 1950s. Back then, when BAT principally sold cigarettes, the focus was predominantly on the chemical and toxicological evaluation of cigarette smoke. With the advent of improved analytical techniques, novel spectroscopic methods enabled the accurate identification of individual compounds in cigarette smoke, even those present at very low (micro-or nano-gram) levels.
Biology advanced with the creation of novel laboratory methodologies that could assess the toxicological impact of cigarette smoke while in parallel, biotechnology and specifically molecular biology techniques were applied to mapping the tobacco genome. The first clinical studies were conducted in the 1980s and 1990s to assess the pharmacokinetics and pharmacodynamics of novel tobacco products and with the advent of novel Tobacco Heating products, more sophisticated clinical studies were conducted to enable the measurement of Biomarkers of Exposure.

1953
Reynolds R&D established

1956
Southampton R&D established

1960s
Chemistry dominates

1970s
Greater focus on smoking behaviour and biology
1980s
Biotechnology embraced

1990s
Increasingly sophisticated analytical techniques to measure smoke constituents
2000s
Advances in biology: biomarkers, diverse cell and tissue systems

2010s
Major focus on reduced-risk products

2011
BAT and Reynolds R&Ds begin to collaborate

2017
BAT acquires the remaining share of Reynolds in 2017
Today’s multi-disciplinary R&D builds on the strong legacy of those origins, where we conduct research using multiple core scientific disciplines, aligned to our scientific assessment framework (Figure 1).
This approach to our transformation has married recruiting new talent from other industries with specific new skillsets, alongside a focussed technical capability development program of our longer-tenure scientists.
Albert Einstein
Figure 1. Our Science Capabilities and our 9-Step Risk Assessment Framework
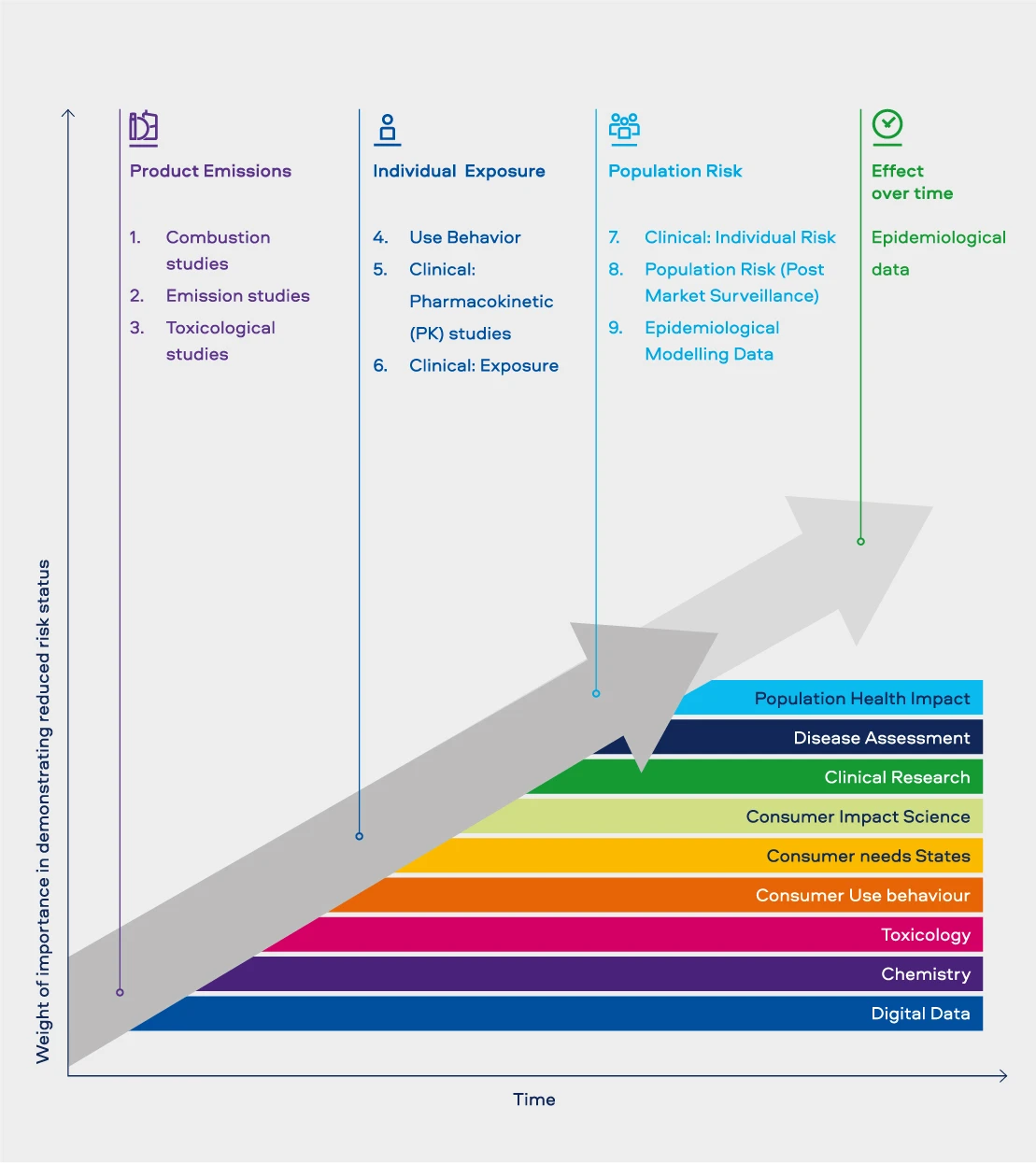
Figure 2. Science Capabilities Architecture
Chemistry
Thermodynamics
Combustion Chemistry
Untargeted Analysis
Mainstream Aerosol Components
Harmful and Potentially Harmful Constituents
Sidestream Aerosol Components
Environmental Aerosol Analysis
Chemometrics
Toxicology
Quantitative Risk Analysis
Physiological Based
Pharmacokinetic [PBPK] Analysis
Toxicology
Mutagenicity
Cytotoxicity
Genotoxicity
Systems Biology
21st Century Toxicology
Organ on a Chip
Clinical Research
Pharmacokinetics
Pharmacodynamics
Abuse Liability
Biomarkers of Exposure
Safety Monitoring
Disease Markers
Quality of Life
Biomarkers of Compliance
Clinical Research
Ethics
Disease Assessment
Adverse Outcome Pathways
Inflammation
Endothelial Function
Lipid Metabolism
Oxidative Stress
Clotting
Lung Function
Population Health
Impact
Real World Data
Real World Evidence
Dynamic Population Modelling
Consumer Risk Perception Studies
Smoker Migration
Longitudinal Consumer
Behavioural Analysis
Consumer Intentions and Perceptions
Epidemiology
Consumer Assessment
Neuroscience
Bioactive Pathway Analysis
Receptor Analysis
Calcium Imaging
Genomics
Need State Methodologies
Consumer Use Behaviour
Human Factors
Topography
Session Consumption
Daily Consumption
Product Preferences
Actual Use
Consumer Switching
Health Wearables
Consumer Impact Science
Chemosensory
Olfactory Science
Gustatory Science
Material Odour
Human Odour Assessment
Teeth Staining
Skin Staining
Indoor Air Quality
Digital Data
Data Standards
Statistical Analysis
Scientific Computing
Sparce Data
Algorithms
Meta Analysis
Artificial intelligence
Actual Use
Machine Learning
We utilised the following ethos as we built our technical capabilities (Figure 2):
Lastly, Digital Data was one discipline that was required across all of the capabilities. A look at the evolution of the data landscape, at how technology is helping businesses solve for today, and how the data analysis that’s possible today can provide us with a new understanding of how we can perform better in the future. Our ethos in augmenting our expertise on Digital Data is based on continuing to build expertise in the classical fields of statistical modelling and analytics, to bringing in novel techniques, which enable the analysis of sparse data and the creation of machine learning systems.
Our ethos in R&D is one of continual improvement where we strive for quantum leaps in our capabilities, but also continually seek the small percentage gains. We envision that advancing our technical capabilities will continue as one of the most important factors in developing and assessing the impact of Smokeless Products on global public health.
Disclaimers
* Based on the weight of evidence and assuming a complete switch from cigarette smoking. These products are not risk free and are addictive.
† Our products as sold in the U.S., including Vuse, Velo, Grizzly, Kodiak, and Camel Snus, are subject to FDA regulation and no reduced-risk claims will be made as to these products without agency clearance.











