Combusting Tobacco & Cigarette Smoke
Table 1. Internationally-standardised puffing parameters
| Puffing Parameter | ISO Regime [1] | Massachusetts Regime | HCI Regime [2] |
|---|---|---|---|
| Puff Volume (mL) |
35 | 45 | 55 |
| Puff Duration (s) |
2 | 2 | 2 |
| Puff Frequency (s) |
60 | 30 | 30 |
| Ventilation Blocking (%) |
0 | 50 | 100 |
Under the 2001 EU Tobacco Products Directive maximum yields for cigarettes were set for tar (10mg/cigarette), carbon monoxide (10mg/cigarette), and nicotine (1mg/cigarette).
We measure our cigarette smoking yields using automated smoking machines (as above), following internationally-standardised puffing parameters (Table 1). These puffing parameters ensure quantitative measurements of cigarette smoke are carried out consistently.
Upon being lit, the tip of a cigarette becomes so hot that, at its peak, it reaches a temperature of around 950°C.[6] The resulting combustion leads to the tobacco breaking down into thousands of chemicals, many of which are not present in the unburnt material.
At these temperatures, oxygen from the air reacts with carbonised tobacco producing simple gases such as carbon monoxide, carbon dioxide and hydrogen.[7] Many of the toxicants found in smoke are formed by the high temperature degradation of tobacco, both through combustion and through pyrolysis (the decomposition of a substance by heat).[8] This is where many of the organic chemicals, including many of the toxicants, found in smoke but not present in the original tobacco are formed.[9]
In cigarette smoke[3,4,5]
>7,500
Individual chemicals present
150
Chemicals known to be harmful
>60
Carcinogens
Some toxicants, such as heavy metals present in tobacco, are not changed by heat and will be either carried in the smoke or will remain in the ash that is produced.
At lower temperatures, typically below 200°C, volatile chemicals in the tobacco, flavour compounds used as ingredients, and nicotine are released into the smoke in a process called distillation.[10]
Puffing: 700-950°C
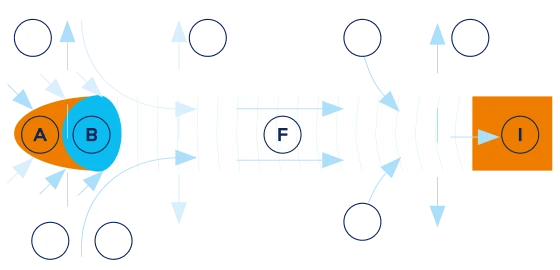
A. Combustion zone
B. Pyrolysis and distillation zone
C. Sidestream gasses
D. Natural convection stream
E. Sidestream smoke
Smoulder: 600-700°C
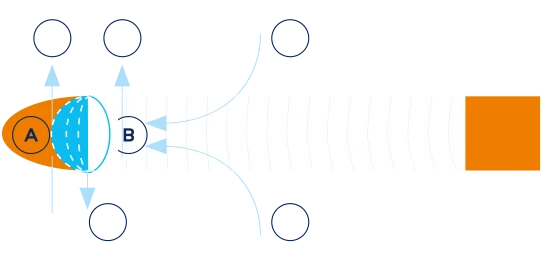
F. Condensation and filteration
G. Light gasses diffusing out
H. Air diffusing in
I. Mainstream smoke
J. Burning coal shaping
Mainstream and sidestream cigarette smoke
The chemical composition of mainstream smoke (the smoke inhaled by a smoker) is different from that of sidestream smoke (the smoke emitted into the environment at the lit end of a cigarette) because of the difference in the temperatures involved in producing the two types of smoke.
Mainstream smoke consists of more than 7,500 individually-identified chemicals. Many of these originate from the tobacco plant, and the amounts of some of them will vary depending on the variety of tobacco used, the soil conditions during growing, and the tobacco curing process.
Sidestream smoke is released at the lit end of a cigarette in a plume and is the main contributor to environmental tobacco smoke. It is mainly formed between puffs at lower temperatures, and under lower oxygen levels than during the combustion that occurs during a puff. Some of its constituents are higher than in mainstream smoke; others lower, and some occur in different phases. For example, in mainstream smoke, nicotine occurs both in the particulate and vapour phases of smoke. In sidestream smoke, nicotine primarily occurs in the vapour phase.[11,12]
An understanding of these processes helps to explain why emissions of Smokeless Products such as Heated Products and Vapour Products are expected to produce aerosols with substantially fewer and lower levels of toxicants than cigarette smoke.
Sources
[8] Oxford English Dictionary online, (Accessed: 28 June 2024)
Sign up for more exclusive the Omni™ content
-
 Smokeless Products: An Introduction
Smokeless Products: An Introduction -
 01. Our Vision: A Need for Reappraisal
01. Our Vision: A Need for Reappraisal -
 02. The Big Questions
02. The Big Questions -
 03. Impact of Smoking
03. Impact of Smoking -
 04. Our Smokeless Science
04. Our Smokeless Science -
 05. Our Smokeless Products
05. Our Smokeless Products -
 06. THR: A Global Transformation
06. THR: A Global Transformation -
 07. THR: Global Regulation
07. THR: Global Regulation -
 08. THR: Scientific Engagement
08. THR: Scientific Engagement -
 09. Our Future Outlook
09. Our Future Outlook -
 10. References
10. References -
 11. Our Published THR Science
11. Our Published THR Science
