

Almost twenty years ago, I joined BAT as an R&D Management Trainee and the BAT I joined then was a very different company from what it is today.
Our ambition is to reduce the health impact of our business, and this is front and centre of our corporate vision to create A Better Tomorrow™ by Building a Smokeless World.[1] This approach is underpinned by Tobacco Harm Reduction (THR). First described by the Institute of Medicine in the U.S.,[2] THR involves the complete switching^ of adult smokers who would otherwise continue to smoke combustible tobacco products like cigarettes, to lower risk profile Smokeless Products.
Harm reduction refers to policies, programmes and practices that aim to minimise the negative health and social impacts associated with the use of certain harmful products.

"I am pleased to introduce our science and innovation resource, Omni™."
Dr James Murphy
Director, Research & Science
Today, if I were to describe BAT in one word, it would be ‘transformation’. We are at the vanguard of the development and scientific assessment of a range of innovative Smokeless Products. We now have three global brands for our portfolio of Smokeless Products: glo (Heated Product), Vuse (Vapour Product) and Velo (Oral Nicotine Pouches). Our Smokeless Products are free from the combustion associated with cigarettes and thus have substantially (>90-99%) reduced levels of a number of toxic chemicals, which are believed to be the primary cause of smoking-related diseases.[3,4,5],*†‡
Our transformation parallels that of many other legacy combustion-based industries (e.g. energy, automotive), which are today transforming their businesses to non-combustion products. In all cases, the combustion of organic material produces toxic chemicals which then have a significant, negative impact on society.
Transforming industries that move from combustion to non-combustion can have a profound benefit to humankind and the environment.
We launched our first Smokeless Products in both the UK and the U.S. over a decade ago, and we now have 29.1 million adult consumers of Smokeless Products.[1]
Our Chief Executive recently re-articulated our Group strategy with clear goals and milestones:[1]
Proof that these metrics and milestones are achievable exist today in Sweden, where (i) Sweden has the lowest lung cancer rates in the whole of the EU as a result of (ii) Sweden's smoking rates being the lowest in the EU (5.6%) as adult smokers have transitioned to Smokeless Products, such as Oral Tobacco Products and Oral Nicotine Pouches.[6]
Sweden is on target to meet the EU’s ‘Beat Cancer 2040 plan’ a full 16 years ahead of the deadline. It’s been reported that if Sweden’s mortality rates were applied to the rest of the EU, there would be 3.5 million fewer deaths over the next decade;[6] an outcome we believe public health and regulators should seek. Finally, (iii) in Sweden, BAT’s revenues from Smokeless Products are far greater than from cigarette sales, dispelling the myth that we wish only to sell cigarettes.[7]
If we could replicate the Swedish experience in all of our global markets it would be a win-win-win for adult consumers, stakeholders and BAT itself.
Over the course of the Omni™, we will show that world-class objective science is crucial to providing answers to the Big Questions (Pages 16-17).
THR requires multi-stakeholder collaboration: regulators need science to form the basis of regulation and public health scientists require it to assess population health impact.
Tobacco and nicotine science is necessary as regulators require specific data on the manufacturers’ products as seen in, for example, the U.S. with Premarket Tobacco Product Application (PMTA) requirements or in the EU with the Tobacco Products Directive (TPD).
Independent science is also required from other stakeholders whether it’s from public health authorities, tobacco control, or regulators.

"I understand there may be scepticism around THR and its science and thus we will endeavour to utilise the Omni™ to demonstrate the totality of THR scientific evidence from a range of institutions and BAT and respond to many of the Big Questions from our stakeholders."
Dr James Murphy
Director, Research & Science
Figure 1: An illustrative model of THR potential
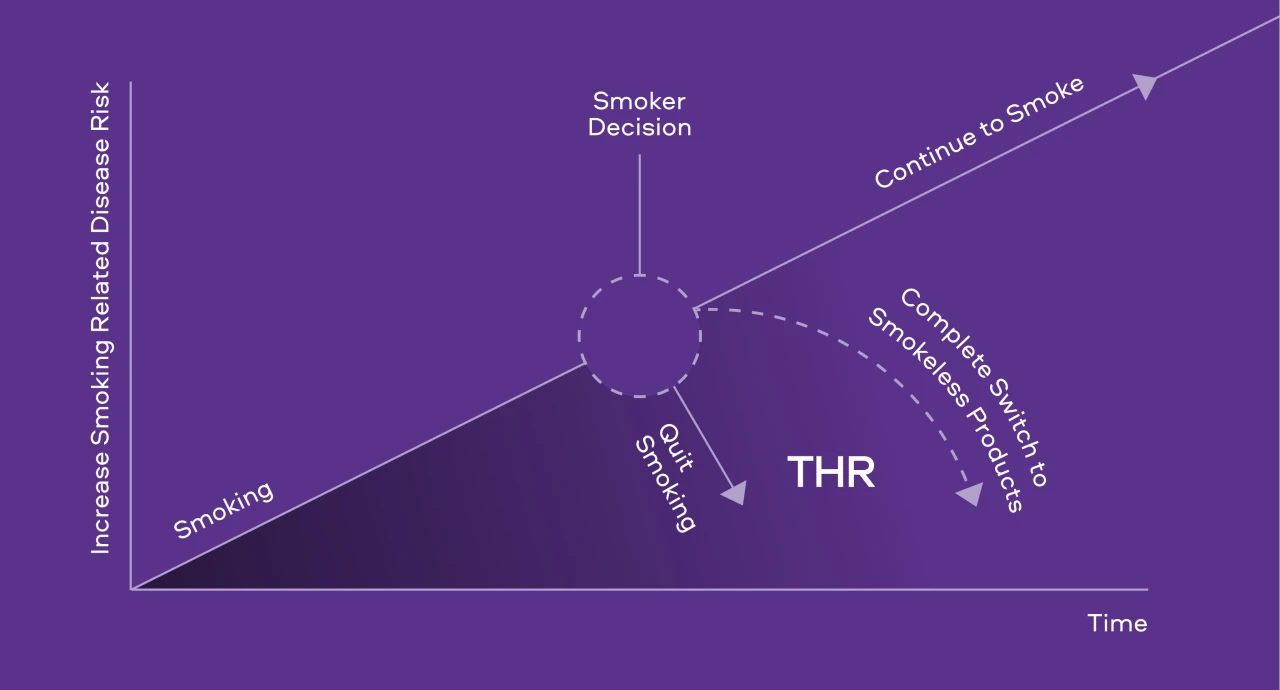
Unfortunately, there remain few scientific forums where all stakeholders (industry included) can discuss scientific evidence and come to common conclusions on THR science to drive progress forward.
We urgently need a major regulator/public health stakeholder to take the lead and offer to chair forums where all parties can participate in an active dialogue on THR.
My hope is that one day very soon we will have multi-stakeholder science-based discussions on tobacco and nicotine products, which would result in informed communications to facilitate the migration of adult smokers who do not wish to quit to lower risk profile Smokeless Products, in turn providing what can be expected to be a beneficial outcome on public health.
The public health message for tobacco use doesn’t change…
“If you don’t use tobacco, don’t start. If you use tobacco the safest thing to do is quit.”
If adult smokers do wish to continue to use tobacco and/or nicotine they should do so with scientifically-proven, lower risk profile products. Tobacco Harm Reduction offers a method of using non-combustible forms of tobacco and nicotine with the potential of lowering an individual’s disease risk, resulting in a net public health benefit.
Footnotes
* Based on the weight of evidence and assuming a complete switch from cigarette smoking. These products are not risk free and are additive.
† Our products as sold in the U.S., including Vuse, Velo, Grizzly, Kodiak, and Camel Snus, are subject to FDA regulation and no reduce-risk claims will be made as to these products without agency clearance.
‡ Comparison with smoke from a scientific standard reference cigarette (approximately 9mg of tar) in terms of the average of the 9 harmful components the World Health Organization recommends to reduce in cigarette smoke.
# The number of consumers of Smokeless Products is defined as the estimated number of Legal Age (minimum 18 years) consumers of the Group’s Smokeless products - which does not necessarily mean these consumers are solus consumers of these products. In markets where regular consumer tracking is in place, this estimate is obtained from adult consumer tracking studies conducted by third parties (including Kantar). In markets where regular consumer tracking is not in place, the number of consumers of Smokeless Products is derived from volume sales of consumables and devices in such markets, using consumption patterns obtained from other similar markets with adult consumer tracking (utilising studies conducted by third parties, including Kantar).
^ While we are aware of some evidence suggesting that dual use of cigarettes with Smokeless Products can, for some smokers, function as a step towards completely switching, we are clear that smokers should not delay making a complete switch.
References











