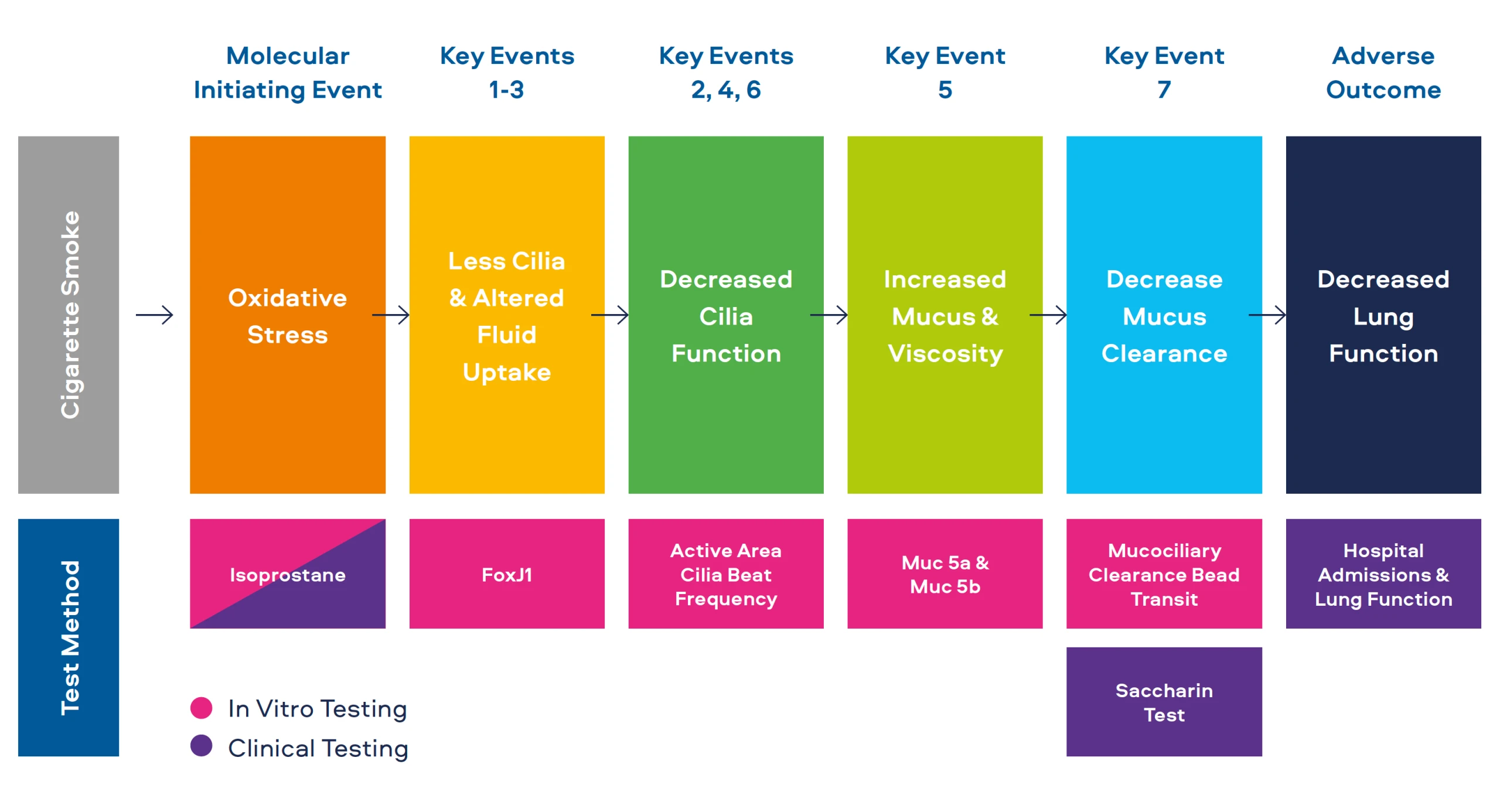
Adverse Outcome Pathways
Adverse outcome pathways (AOPs) were first proposed by the Organisation of Economic Co-operation and Development (OECD), with the goal of reducing the need for animal testing.[1] AOPs describe how an exposure to a stimulus can produce a series of changes resulting in a disease or adverse outcome (AO).

"The adverse outcome pathway (AOP) has been proposed as a simplified organisational construct to contribute to this transition by linking molecular initiating events and earlier (more predictive) key events at lower levels of biological organisation to disease outcomes."
Anna Bal-Price
Developmental Biologist
European Commission Joint Research Centre[5]