FDA Regulations
The U.S. Food and Drug Administration (FDA)’s Center for Tobacco Products regulates the manufacturing, marketing, and distribution of tobacco products.
The FDA’s authority was originally limited to cigarettes, smokeless tobacco, and roll-your-own tobacco. However, in August 2016, it issued a regulation deeming other Smokeless Products, such as Heated Products, Vapour Products and Oral Nicotine Pouches, to be tobacco products under the agency’s authority.
The FDA has recognised the principles of harm reduction, and it weighs the health impact on the public as part of its Premarket Tobacco Product Application (PMTA) process. Additionally, the Modified Risk Tobacco Product (MRTP) process is specifically designed to recognise reduced-risk and reduced-exposure products, by authorising the use of product claims to alert adult consumers to products considered to be of reduced exposure and/or risk, so that they might consider switching.
FDA's Regulatory Process
Smokeless Products, including Heated Products, Vapour Products, Oral Tobacco Products, and Oral Nicotine Pouches, are subject to various statutory and regulatory requirements, including the following:
- Premarket authorisation, including existing products (that have been modified or that were not previously authorised), and future entrants to the market;
- Ban on sales to those under 21 years of age;
- Requirements to place text health warning statements on packaging and advertising; and
- Requirements that manufacturers submit ingredient information and product listings to regulatory authorities.
Premarket review requirements
All tobacco and non-medicinal nicotine-containing products not on the market prior to 15 February 2007 require FDA authorisation (‘pre-market review’) in order to stay on (or enter) the market.
For new-to-market products, manufacturers must submit a PMTA,[1] which must contain sufficient scientific evidence to demonstrate that a product is "appropriate for the protection of the public health." The FDA evaluates the evidence provided to determine whether the potential risks and benefits of the marketing of a product would have a net positive effect on the health of the population, including tobacco product consumers as well as non-consumers. The intention is to prevent the introduction of tobacco products that result in net harm to the population.
Even improvements to devices, such as for safety, ease of use, toxicity, and consistency of delivery, cannot be introduced without a PMTA filing and related marketing order.
Tobacco-flavoured Vuse Solo, Vuse Ciro, Vuse Vibe, and Vuse Alto have all received Market Granted Orders (MGOs) through the PMTA process

Figure 1. Premarket Tobacco Product Application (PMTA) Status [2]
PMTAs received by FDA
26,607,739*
Number of Market Denial Orders (MDOs)
1,263,860*
Combined number of PMTAs refused acceptance and refused filing
24,726,289*
Number of Market Granted Orders (MGOs)
44**
Percent of MGOs for devices
27%
Percent of MGOs for consumables
Heated Products
14%
Vapour Products
52%
Percent of Heated Product Consumable MGOs, Tobacco Flavoured
67%
Percent of Heated Products Consumable MGOs, Menthol or Non-Tobacco Flavoured
33%
Percent of Vapour Products Consumable MGOs, Tobacco-Flavoured
85%
Percent of Vapour Products Consumable MGOs, Menthol or Non-Tobacco Flavoured
15%
* Note: Totals reflect the FDA’s latest metrics covering Oct. 2019 – Mar. 2024 for Heated Products and Vapour Products PMTAs
** Total from 1 October 2019 to 31 July 2024. FDA, Searchable Tobacco Products Database, https://www.accessdata.fda.gov/scripts/searchtobacco/, accessed 3rd August 2024.
Modified Risk Tobacco Product requirements
The 2009 Family Smoking Prevention and Tobacco Control Act introduced the term ‘Modified Risk Tobacco Product’ (MRTP), defined as: “any tobacco product that is sold or distributed for use to reduce harm or the risk of tobacco-related disease associated with commercially marketed tobacco products.”[3]
Manufacturers may apply to the FDA for a modified risk order, or a modified exposure order, which necessitates the submission of a vast amount of supporting material – epidemiological data, biomarker data, preclinical toxicology, projected behaviour, marketing impact, and more – to support the application.
Successful applications allow for reduced risk or reduced exposure claims to be made on specific brand variants of tobacco or nicotine-containing products for a time-limited period, subject to ongoing post-market review and reporting requirements. Approved claims can then be placed on advertising materials as well as on packs, as specified in the modified risk order issued by the FDA.
The U.S. MRTP process is a risk and claim substantiation process with specific provisions that allow for the formal recognition of reduced risk and exposure. To date, few products have received MRTP Granted Orders.[4]

FDA’s Regulatory Process: Our Views
We support the principles behind the PMTA and MRTP processes in that they acknowledge that the marketing of Heated Products, Vapour Products and Oral Nicotine Products in principle can be considered as "appropriate for the protection of the public health" as compared to combustible cigarettes. Any decision regarding the reduced risk and safety of Smokeless Products should be evidence-based and scientifically substantiated. Products that are proven to reduce risk should have marketing and product freedoms in line with their risk.
The PMTA process presents a huge opportunity to improve public health by Tobacco Harm Reduction. However, we believe that the process is not yet working as intended—not for the FDA, adult consumers, or manufacturers. The administrative systems in place can be overly burdensome, for both the industry and the regulator, and can constrain innovation by requiring new authorisation for any changes to previously authorised products. Additionally, insufficient enforcement of the relevant laws has allowed dangerous and un-stewarded illegal products to remain on the market. There has been such an influx of unauthorised Vapour Products that the number of different e-cigarette devices sold as nearly tripled to more than 9,000 since 2020, driven by these unlawful products.[5]

"The FDA’s PMTA process should promote—rather than hinder—innovation of potentially less risky Smokeless Products. The FDA should foster innovation to ensure that a greater number of potentially Reduced-Risk Products are made available to adult smokers. This will in turn serve to accelerate Tobacco Harm Reduction and a transition to a smokeless future. At the same time, meaningful enforcement should remain a priority, to curb the continued growth of illicit, unregulated, and underage-appealing products that continue to flood the marketplace."
Tim Nestor
Executive Vice President, U.S. R&D
RAI Services Company
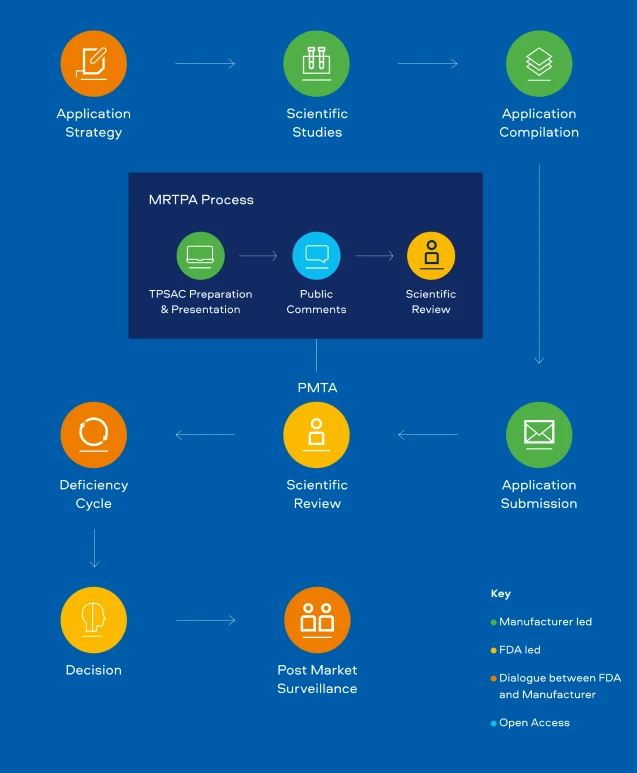
PMTA & MRTPA Process
References
[1] U.S. Food and Drug Administration, Premarket Tobacco Product Applications. (Accessed: 26 July 2024)
[2] U.S Food and Drug Administration, PMTA Acceptance Phase Metrics. (Accessed: 31 July 2024)
[4] U.S Food and Drug Administration, Modified Risk Granted Orders. (Accessed: 31 August 2024)
[5] AP News, Thousands of unauthorized vapes are pouring into the US despite the FDA crackdown on fruity flavors. 2023. (Accessed: 26 July 2024)
Sign up for more exclusive the Omni™ content
-
 Smokeless Products: An Introduction
Smokeless Products: An Introduction -
 01. Our Vision: A Need for Reappraisal
01. Our Vision: A Need for Reappraisal -
 02. The Big Questions
02. The Big Questions -
 03. Impact of Smoking
03. Impact of Smoking -
 04. Our Smokeless Science
04. Our Smokeless Science -
 05. Our Smokeless Products
05. Our Smokeless Products -
 06. THR: A Global Transformation
06. THR: A Global Transformation -
 07. THR: Global Regulation
07. THR: Global Regulation -
 08. THR: Scientific Engagement
08. THR: Scientific Engagement -
 09. Our Future Outlook
09. Our Future Outlook -
 10. References
10. References -
 11. Our Published THR Science
11. Our Published THR Science
